LEO Pharma delivered on strategic ambitions and showed resilience during COVID-19 pandemic in 2020
Strategic psoriasis products Enstilar® and Kyntheum® continued to grow and gain market share. Total revenue declined 1.8% when excluding discontinued or divested products. This was offset by the impact of coronavirus lockdowns, generics in Europe and further price pressure in the US.
- Revenue of Enstilar® increased 3% (+10% in Europe) to DKK 1,125 million, compared to topical market declining 2%
- Revenue of Kyntheum® increased 40% to DKK 467 million compared to market growth of biologics for psoriasis of 16%
Strategic projects progressed as planned while LEO Pharma ensured business continuity
In response to the coronavirus pandemic, LEO Pharma successfully implemented a plan to ensure safety and corporate responsibility, continuity of supply, and continuity of strategic projects.
- Supply chain and clinical trials continued uninterrupted
- Introduced 2030 strategy and aligned its organization to meet the ambitions of continuous growth and launch of a first- or best-in-class medicine every 2-3 years
- Divested non-core assets to Cheplapharm
- Committed to the Paris Agreement on climate change by setting a science-based target with a program to reduce CO2 emissions by 50% by 2030.
- Established a GLT with key focus on diversity in gender (50/50), age and competences with focus on delivering performance
R&D pipeline advanced significantly
LEO Pharma’s R&D pipeline progressed and achieved several important milestones, in line with the 2030 strategy.
- Phase 3 results for tralokinumab in atopic dermatitis enabled FDA and EMA filling. Anticipated global launch in 2021
- Announced positive results of a phase 2b dose-finding study with delgocitinib cream in adult patients with moderate-to-severe chronic hand eczema
- New partnership with Oneness Biotech and Microbio Shanghai for atopic dermatitis and asthma
- Added an IL-17 small molecule modulator for treatment of psoriasis from own R&D to the pipeline
EBIT: Operating loss better than financial guidance
Despite a decline in revenue and continued heavy investments in the R&D pipeline and global launches, LEO Pharma exceeded its profitability targets through building and agile, simple and efficient organization and a gain from the divestment of non-core products to Cheplapharm.
Focus in 2021 on launch of tralokinumab, further R&D investments and implementation of lean operating model
In 2021, LEO Pharma will continue to move forward towards realizing its 2030 ambitions by the anticipated launch of tralokinumab and continued investments in clinical development and focus on growth and profitability for the established portfolio. Revenue is expected to remain flat affected by the divesture of non-core products, while the operating loss will grow due to the investment in LEO Pharma’s future products.
Catherine Mazzacco, CEO and President of LEO Pharma commented:
“2020 was an eventful year for all, but I am happy to say that LEO Pharma delivered significant strategic progress and solid financial performance. Our rapid response to coronavirus helped us keep our promise to patients, while meeting our operating profit goals and advancing our strategic projects uninterruptedly. We expanded our market share for the strategic products Enstilar® and Kyntheum® and maintained our position in thrombosis, setting us up well for the anticipated market recovery in 2021. Tralokinumab is under regulatory review in the US and in Europe, and we are looking forward to launching this new treatment option for atopic dermatitis when approved in 2021. It is an important opportunity for LEO Pharma, because patients around the world need additional options. In line with our 2030 strategy which we introduced last year, we will continue to accelerate our R&D efforts to rapidly expand and diversify our portfolio in a range of dermatological indications.”
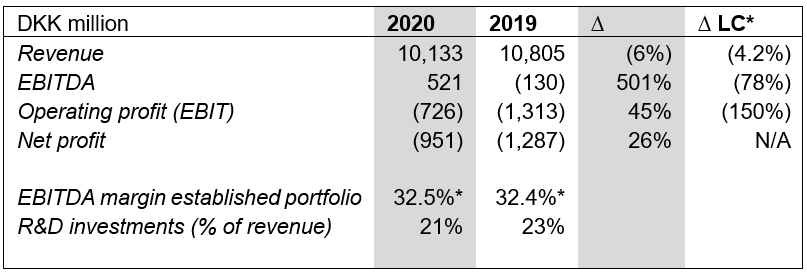
Key figures
Annual report 2020
The full results are available in the LEO Pharma Annual Report 2020 on the company’s website at https://www.leo-pharma.com/Our-annual-reporting
Changes to LEO Pharma’s Board of Directors
At LEO Pharma’s Annual General Meeting, Lars Green of LEO Holding and The LEO Foundation was elected new member of the board. Cristina Lage and Jesper Hoejland did not stand up for re-election and will leave LEO Pharma’s Board of Directors.
About LEO Pharma
LEO Pharma is a global leader in medical dermatology. We deliver innovative solutions for skin health, building on a century of experience with breakthrough medicines in healthcare. We are committed to making a fundamental difference in people’s lives, and our broad portfolio of treatments serves close to 100 million patients in over 70 countries annually. Headquartered in Denmark, LEO Pharma has a team of 4,000 people worldwide. LEO Pharma is co-owned by majority shareholder the LEO Foundation and, since 2021, Nordic Capital. For more information, visit www.leo-pharma.com


