- Home
- Our science
- View our pipeline
Innovate to address patient needs
The challenge of skin disease is immense, and the number of hard-to-treat indivations is staggering. Millions of patients suffer from clinical symptoms for which we have no answers or only answers that fail to advance the quality of their lives.
We are ready to address the challenge. Medical dermatology is an area with untapped potential and attractive opportunities for us to grow. We have the expertise to bring solutions to these underserved conditions.
We are continuously working to strengthen our pipeline with early as well as late-stage assets addressing underserved dermatological disease areas. Ultimately, we aim to make a fundamental difference for those who need us most in medical dermatology.
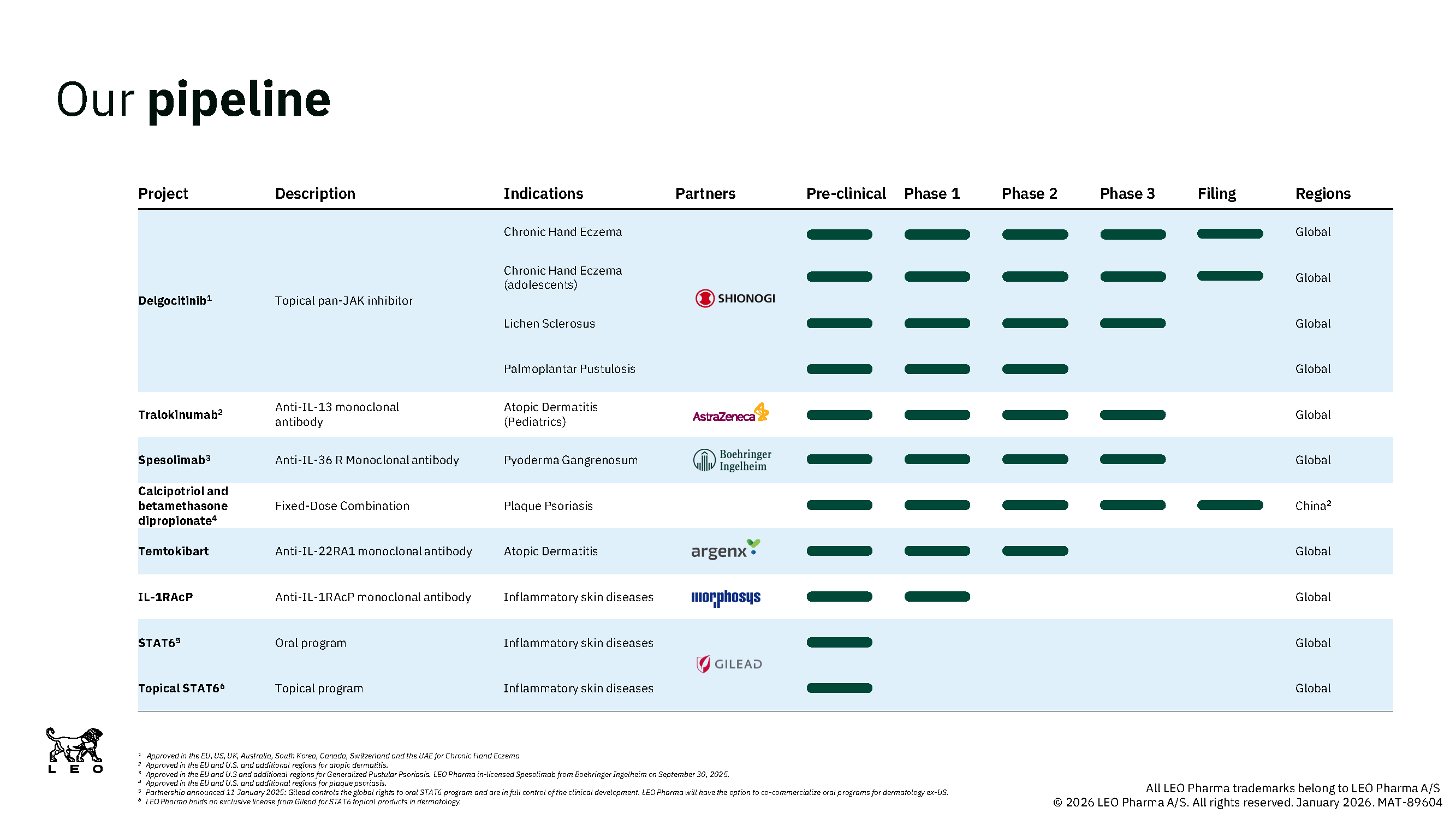
Our current pipeline
The current pipeline represents a strong mix of projects aimed at topical, oral and injectable treatments. (Click the image below to view a larger version)